Corrosion of cathode by plasma of different reactive gases in cathode arc discharge
Cathodic arc discharge has been often used to deposit different metal films. In addition to Ar, which is commonly used for direct sputtering of target materials to deposit corresponding metal films, nitrogen and oxygen containing films are also often obtained by adding O2 and N2 to discharge during deposition. Different from the elemental Ar plasma discharge, N2 and O2 are reactive gases, and the plasma process is more complex, so different discharge forms are formed on the surface of the cathode arc. With the different discharge modes on the surface of the cathode target, the ion corrosion on the surface of the target is also different. In addition to the corrosion morphology of the target surface, the composition and content of the corresponding parts are also very different. Therefore, a detailed study of the corrosion behavior of different discharge gases on the target surface will help us to better understand the performance of plasma on the target surface, such as ion distribution, free radical distribution, and will also help us to have a better understanding of the discharge behavior of the whole cathode arc.
1) Ar,N2 and O2 plasmas exhibit different etching behaviors on the cathode;
2) The elements in different corrosion areas are also significantly different;
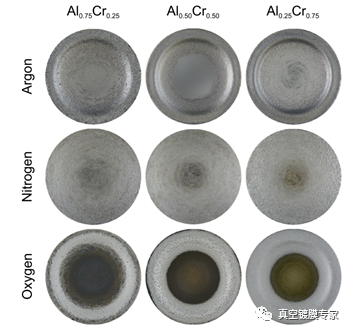
FIG. 1 Corrosion shapes of different discharge gases on the target surface.
As shown in Figure 1, first of all, we can see that different discharge gases have different corrosion shapes on the target surface. For Ar discharge can see obvious corrosion marks, unlike Ar discharge, N2 better discharge etching uniformity, corrosion area bigger, from the center to the peripheral can have better corrosion, for the discharge of O2, you can see the poor corrosion effect, only in the edge of a obvious corrosion ring, can't make good use of the center area. Through these corrosion traces, we can indirectly get the distribution shape of different reaction gas plasma on the target surface.

Figure 2. Element distribution in the corrosion region of Al0.5Cr0.5 (a)Ar discharge, (b)N2 discharge and (c)O2 discharge.
Under the backscattering SEM, we can see that for the corrosion area, N and O distributions are not seen (as shown in Figure 2), indicating that the target surface in the corrosion area is relatively clean, and further indicating that no free radicals infiltrate into the target surface, even if they do, they will be sputtered out in the continuous sputtering. For Ar and O2 discharges, we can observe that Al and Cr distributions are more uniform under N2 discharges, indicating that sputtering particles on the target surface are also more uniform, which is consistent with the phenomenon observed in FIG. 1.

Figure 3. Element distribution of Al0.5Cr0.5 (a) corrosion area under O2 discharge, (b)O2 discharge center area, (c)N2 discharge center area
To further observe whether there is no infiltration of N and O in the corrosion area, further observe the distribution of O in the enlarged area, as shown in FIG. 3(a). It can be observed that only weak oxidation is observed on the surface, but no O distribution is observed in the deep section, indicating that the oxidation in the corrosion area is very weak. Compared with the central region, the oxidation is very obvious, forming Al2O3 on the surface and CrO in the deep layer. For N2, CrN is the main component in the central region.
Through the above study, we can observe the corrosion of different discharge gases on the target surface and the formation conditions, which is helpful to understand the discharge behavior of plasma with different reaction gases on the cathode target surface. But there are some questions that need to be raised based on the above research
1) At present, only the element distribution behavior of some local particles is given in this paper, but the element content is not given, so the specific N and O content cannot be confirmed.
2) Secondly, without XPS analysis, the expression forms of Al-N,Cr-N,Al-O and Cr-O cannot be accurately determined.
1.Erosion behavior of composite Al-Cr cathodes in cathodic arc plasmas in inert and reactive atmospheres
Literature (contributed by xdz)
 18922924269
18922924269

